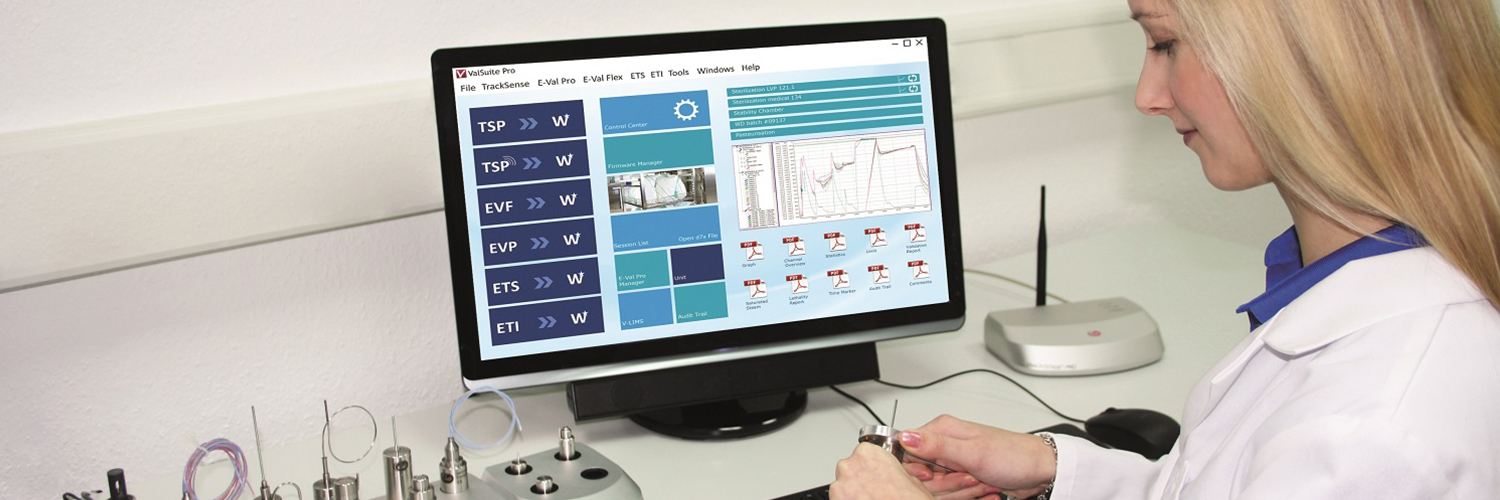
Quality assurance in the processing of laboratory goods
The validation of autoclaves, installations and processes present a special challenge in laboratories. A fundamental requirement for an established quality management system in the laboratory is the furnishing of proof that processes function so that they are transparent and reproducible. The autoclaves or installations have to meet certain requirements depending on the product that is to be sterilised, so that reproducible and evaluable processes can be achieved. The validations serves as proof of the function and performance of the autoclaves within a process.
Our qualified validation team supports you with its know how so that it can together with you, implement the quality requirements both in laboratories that work with biological agents and in manufacturing companies (e.g. the manufacturing of pharmaceutical products, active ingredients, cosmetics.
The validation is hereby based on DIN EN ISO 17665, GLP or GMP respectively and the German Society for Hospital Hygiene (DGKH) Recommendation for the Validation and Routine Monitoring of Sterilisation Processes with Moist Heat for Medical Devices.



